Non-Invasive Detection of Cancer
Circulating Tumor DNA for Targeted Therapy Guidance
While the 5 year survival rate for lung cancer is 55% when detected at an early stage and still confined to the lungs, only a small population of lung cancer patients (16%) are diagnosed at this stage. For the remaining cases, the 5-year survival rate is only 4% as the vast majority of patients do not present themselves until late stages of the disease. In total, 1.6 million people die each year from lung cancer, which is more deaths than caused by colon, breast and prostate cancer combined.
Significant progress in the treatment of non-small cell lung cancer (NSCLC) has been made with the development of therapies that target proteins and pathways responsible for tumor growth. One such target is the epidermal growth factor receptor (EGFR). Mutations have been found in the EGFR gene that indicate whether or not a patient will respond to specific EGFR targeted therapies. While tumor tissue biopsies can be taken and analyzed for the presence of these markers, this procedure is invasive and cannot be performed serially to track cancer-specific mutations over time.
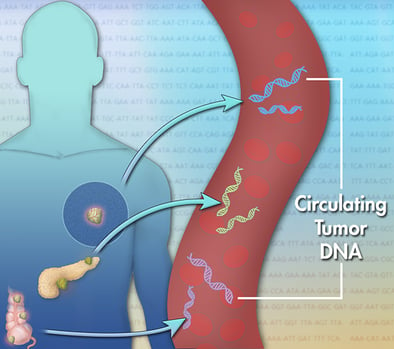
Tumor cells shed small amounts of their genetic material into the blood stream known as circulating tumor DNA (ctDNA). Unlike tissue biopsies, blood can be repeatedly sampled for ctDNA analysis to guide and monitor therapeutic decisions. While there are several available assays for ctDNA, they require that samples be sent to a centralized lab for next-generation sequencing (NGS) or digital polymerase chain reaction (dPCR) analysis which require time-consuming and complex assay protocols, as well as expensive instrumentation. Giner has demonstrated a unique, prototype electrochemical sensor for detection of ctDNA in plasma of NSCLC patients. Giner’s sensor detects T790M and L858R mutations in the EGFR gene that are implicated in the treatment of NSCLC. Our assay has minimal sample preparation requirements and uses a simple and easy-to-operate instrument with an analysis time of 4 hours or less.
These improvements represent significant advances in the treatment and diagnosis of NSLC:
- A non-invasive test that can be performed repeatedly over the course of treatment.
- A rapid, less expensive and easy-to-operate ctDNA assay that can be used at the point-of-care.
- More timely selection of initial therapies, and therapy changes during treatment that may improve patient outcomes.
- Early stage diagnosis will improve patient 5-year survival rates.