Technology
Miniature oxygen generators for life sciences
Delivery of vital oxygen without gas cylinders
 Giner Life Sciences (GLS) manufactures a range of small oxygen generators that enable unlimited oxygen supply for critical applications where portability or small size is at a premium. Oxygen generators can be finely controlled by regulating the current, minimizing need for pressure, oxygen and/or flow measurements.
Giner Life Sciences (GLS) manufactures a range of small oxygen generators that enable unlimited oxygen supply for critical applications where portability or small size is at a premium. Oxygen generators can be finely controlled by regulating the current, minimizing need for pressure, oxygen and/or flow measurements.
Oxygen generators can be of two types:
- Oxygen Generator: converting water into oxygen and hydrogen gas via electrolysis
- Used with our human implant technology
- H2 is either utilized or safely removed
- Oxygen Concentrator: Generating oxygen from air with low/no water requirement
- An option in external wearable and bench top applications
- An electrochemistry combining fuel cell and electrolyzer reactions where air is available and hydrogen generation is not desired


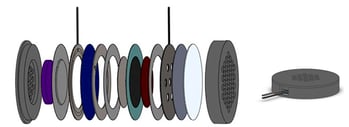
Implantable Oxygen Generators
Oxygen and hydrogen gas generated and delivered inside the body

The Giner Life Sciences iEOG (implantable electrolytic oxygen generator) is capable of producing 5 scch oxygen when drawing 0.02 amps. The device harvests small amounts of water through the water harvesting membrane (in white) to fuel the reaction.
This iEOG has been constructed of implant-grade materials to enable direct implantation into the body. Oxygen and/or hydrogen can be supplied locally for therapeutic applications, such as oxygenated cell capsules or enhanced tissue growth and healing.
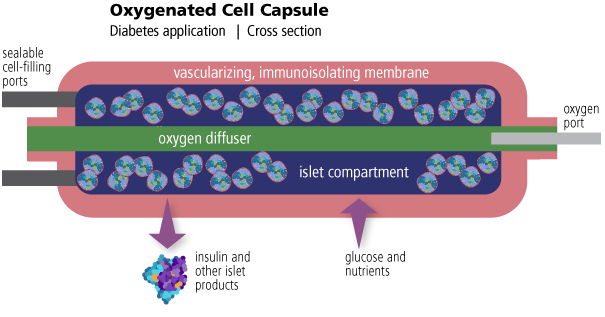
Oxygenated Cell Capsules
Implantable immune-isolating capsules with supplemental oxygen
Giner Life Sciences has developed proprietary cell capsules which can be connected to a supply of oxygen from a GLS implantable oxygen generator (iEOG). Oxygen is diffused at a controlled rate from the center of the cell capsule to supplement the oxygen, glucose and nutrients that are being transported across the immunoisolating layers on the outside of the cell capsule. Our first application in development is the transplant of islet cells for curative therapy of diabetes.