Lithium Sulfur Batteries
Today, everything from electric vehicles to consumer electronics and satellites rely on batteries for energy storage. Lithium ion batteries (LIB) currently dominate the market, having reached their theoretical performance limits. LIBs ability to meet the worlds growing needs is further limited by the scarcity of the raw materials, including Ni and Co, and the environmental impact of mining and processing these materials. To decarbonize the transportation and energy sectors, commercialization of next generation battery chemistries which can double or triple the energy density of LIBs will be required.
Lithium sulfur (Li-S) batteries are considered one of the most promising technologies for next-generation power applications ranging from EV and aerospace applications to consumer electronics. In Li-S batteries, the toxic and expensive cathode materials used in LIBs is replaced with sulfur, which is abundant, inexpensive and has significantly higher specific capacity than state of the art Li-ion active materials. Li-S can also operate safely over a wider temperature range and has been shown to operate over the range of -40°C to 70°C. With 2-3X the energy density of Li-Ion, Li-S batteries provide an opportunity to significantly reduce the size, weight, cost, and environmental impact of battery packs to transform the energy storage landscape.

Polysulfide Blocking Coatings
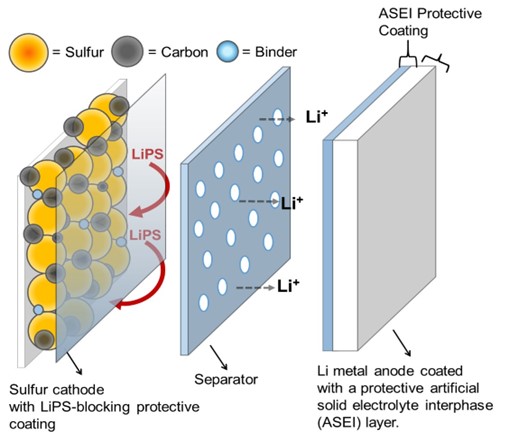
Giner has developed patented coating technology designed to mitigate polysulfide shuttling, the leading cause of low cycle life in Li-S batteries. As sulfur is converted into Li2S during discharge, the reaction proceeds though a series of lithium polysulfides, some of which are soluble in the electrolyte. These soluble polysulfides migrate from cathode to anode through the electrolyte and reacting on the anode, a process known as “polysulfide shuttling.” Polysulfide shuttling leads to side reactions that consume sulfur, electrolyte, and Li, resulting in rapid capacity decay, low cycle-life, and poor round-trip efficiency.
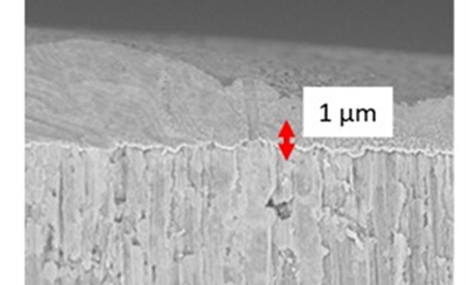
Giner Labs coatings have been developed for anode, cathode and separator applications and have demonstrated the ability to block polysulfide migration through both physical and chemical mechanisms. These coating provide both mechanical and chemical protection to the anode against polysulfide shuttling, as well as self-healing properties to maintain this protection through repeated dimensional changes.
Electrode Development
Cathode design is critical for achieving cycle life targets for Li-S batteries. Sulfur is an electrically insulating material. During cycling, sulfur is converted through a series of insoluble (solid) and soluble polysulfide species, resulting in a final Li2S discharge product and an 80% increase in volume compared with sulfur. During this process, sulfur can migrate within the cathode, and if uncontrolled, can result in reduced conductive reaction sites and isolated sulfur deposits. Additionally, volume changes within the cathode can lead to broken electronic pathways, isolating whole areas of the cathode. To support cycling, the cathode design must maintain an electronically conductive framework to supply electrons to complete the reaction process, ionic pathways through electrolyte for Li+ to reach the active sites, and mechanical stability to withstand the volumetric changes on both the anode and cathode during the full cycling process.
Giner Labs has developed a Li-S cathode to enable high sulfur content of >64 wt % sulfur demonstrating >90% round trip efficiency for >100 cycles at practical loadings of ~4 mg S/cm2. Our cathode design utilizes an environmentally safe binder system which eliminates the toxic solvents and fluorinated polymers common in other electrode designs. This reduces cost and exposure to toxic solvents during the electrode fabrication process.
Currently, ongoing programs include further optimization of the cathode composition to increase sulfur content, immobilize sulfur and facilitate fast, efficient sulfur conversion during cycling.