Personal Monitoring: Real Time Tracking of Bio Attributes for Remote and Home Use
Wearable Monitoring Sensors
It started with Smart watches and health apps, and now remote personal monitoring has a large market share in the diabetes industry with continuous glucose monitors (CGMs). Personal monitoring will play a pivotal role in home and wearable diagnostics in the future markets, and will fill a big need and demand.
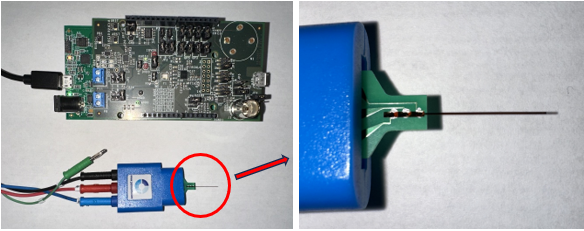
Current wearable alcohol monitors are not accurate and are prone to tempering. Giner’s subcutaneous continuous alcohol monitor (CAM) will be the first commercial wearable device for accurate and reliable and discreet recording and reporting of blood alcohol levels. Most importantly, Giner is using this project to build a Common Wearable BioSensor Platform, allowing Giner to become an OEM Developer of various wearable sensors, with the general sensor design, deployment, and patient mobile portals for other analytes requiring minimal changes.

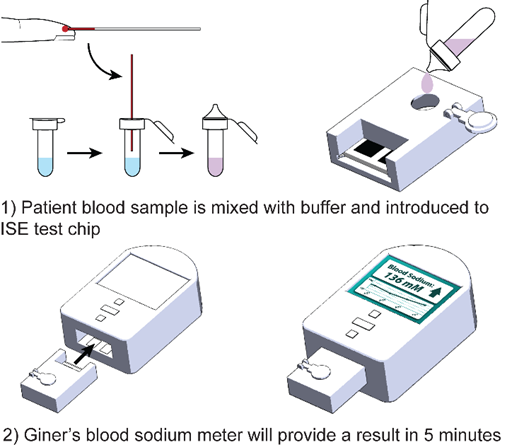
At-Home Blood Sodium Test
Arginine Vasopressin Deficiency (AVP-D), caused by a deficiency of the hormone vasopressin, is a rare disease that effects the body’s ability to regulate sodium in the blood. This can cause severe dehydration and leads to frequent hospitalizations for individuals allected by the disease. Currently, individuals with severe AVP-D travel to labs frequently (1-4 times per week) to have venous blood drawn and the lengthy turnaround times lead to preventable complications from mismanagement of AVP-D. Giner is developing a blood sodium test that will utilize a modified Ion Selective Electrode (ISE) methodology for in-home use. This technology will provide a rapid, accurate, and low-cost home test to conduct routine blood sodium measurements. Similar to a blood glucose test, it will rely on capillary blood samples (<90 µL, 2-3 drops), unlike current sodium tests which require venous blood or 20-30 drops of capillary blood and are not FDA approved or covered by insurance for home use.
Bedside Nutritional Monitoring of Human Milk
For infants, growth in the first weeks of life is critical to optimal lifelong growth and development. The American Academy of Pediatrics (AAP) has recommended that all preterm infants receive mother’s milk or banked human donor milk if mother’s milk is unavailable or in low supply. Although human milk (HM) is considered the optimal nutrition for all infants, it has tremendous variation in composition especially for total protein concentration. Protein supplementation beyond this standard amount is initiated once an infant’s growth falters, at which point both somatic and brain growth may have already been compromised. Neonatal intensive care units (NICUs) are limited in their ability to individually, custom-fortify HM, as there is no bedside point-of-care (POC) tool available to measure HM macronutrient content on an hourly basis, limiting the care giver’s ability to provide optimal nutrition to the vulnerable preterm infant.
Based on the initial success of preliminary results, Giner Labs is developing an electro-chemical POC protein assay for human milk and for accurate and quantitative detection of human milk protein level using only two drops of sample. We envision that our first market entry will be a fully developed POC test that works like a bedside blood glucose test, where based on immediately available results, a nurse can fortify the human milk prior to feeding the infant.

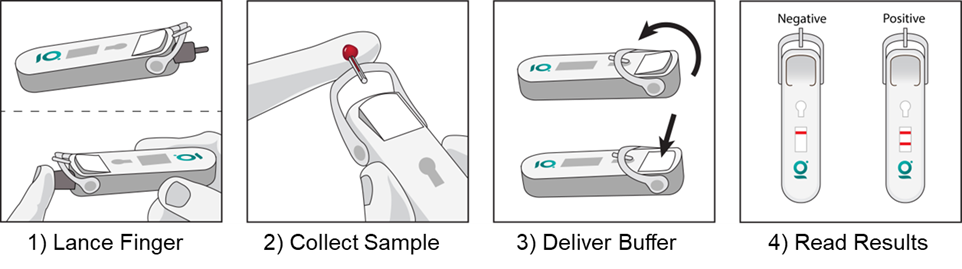
Over-the-Counter Test for Home Screening of Prostate Cancer
Prostate cancer is the second most diagnosed cancer in men and remains one of leading causes of cancer mortality for men. Prostate Specific Antigen (PSA) is the most reliable and mostly accepted biomarker for screening and monitoring of prostate cancer status. Elevated PSA levels may not conclusively diagnose cancer, but they are the first warning sign of the onset of cancer, and they warrant further confirmation using MRI imaging and then biopsy. While the incidence of prostate cancer has declined in general over the years, the incidence and mortality rate among African American men is still disproportionately high, and a reliable home test will help both underserviced and remote communities. Giner has applied for funding with the National Cancer Institute to develop an easy-to-use self-test for PSA analogous to home COVID-19 testing, that will use capillary blood obtained from a finger stick. In collaboration with Atomo Diagnostics (San Diego, CA), Giner will leverage its Lateral Flow Assay (LFA) test with an innovative user-centered platform for capillary blood collection (AtomoRapid™ Pascal) to successfully deliver a home self-test device, which can be operated in three simple steps. The proposed LFA test will have a positivity PSA threshold of 10 ng/mL, will ensure usability by the target population of males over 40 years old, and provide a rapid result (less than 15 minutes).